Abstract
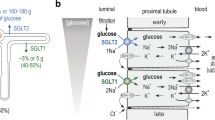
In order to study contraluminal hexose transport, concentration and time-dependent influx of3H-2-deoxy-d-glucose from the interstitium into cortical tubular cells has been measured. The influx curves fit to a two parameter kinetics (K m 1.3±0.2 mmol/l,J max 0.67±0.16 pmol/s · cm) plus an additional diffusion term (withP=6·10−8 cm2/s) and a distribution ratio extracellular to intracellular amount of 2-deoxy-d-glucose of 1∶0.6. Since the extracellular to intracellular free water space as estimated from morphological data was 1∶2, one must conclude that glucose has only free access to 1/3 of the cell water. The intracellularly accessible space was augmented when the tubules were preperfused for 10 s with hypotonic saline. Thereby an increase of the compartment into which diffusion occurs was revealed and a final rupture of this intracellular compartment at 1/4 isotonic solutions was observed. Total replacement of ions in the peritubular perfusate by mannitol did not change 2-deoxy-d-glucose influx, indicating that it is Na+-independent. By adding isotonic concentrations of the respective sugars to the capillary perfusate, three degrees of inhibition of 2-deoxy-d-glucose influx could be revealed: strong inhibition byd-glucose, methyl-β-d-glucoside,d-mannose, 3-O-methyl-d-glucose, 2-deoxy-d-galactose, methyl-β-d-galactoside and 6-deoxy-d-glucose, moderate inhibition byd-galactose,l-glucose,l-mannose andd-fructose, no or borderline inhibition by methyl α-d-glucoside, 2-deoxy-methyl-α-d-galactoside, 1-thio-β-d-glucose, 1-thio-β-d-galactose, 5-thio-α-d-glucose, myo-inositol and mannitol. The contraluminal 2-deoxy-d-glucose influx was also inhibited by phloretin, chlormerodrin and preperfusion with cytochalasin B. Starvation as well as streptozotocin diabetes has no influence on contraluminal 2-deoxy-d-glucose transport. Thus, in contrast to the luminal hexose transport system the contraluminal system is Na+-independent, does not require on OH-group at C-atom 2, acceptsl-glucose and fructose, but not an α-methyl group at C-atom 1.
Similar content being viewed by others
References
Anchors JM, Haggerty DF, Karnovsky ML (1977) Cerebral glucose-6-phosphatase and the movement of 2-deoxy-d-glucose across cell membranes. J Biol Chem 252:7035–7041
Aronson PS, Sacktor B (1975) The Na+ gradient-dependent transport ofd-glucose in renal brush border membranes. J Biol Chem 250:6032–6039
Barfuss DW, Dantzler WH (1976) Glucose transport in isolated perfused proximal tubules of snake kidney. Am J Physiol 231:1716–1728
Basketter DA, Widdas WF (1978) Asymmetry of the hexose transfer system in human erythrocytes. Comparison of the effects of cytochalasin B, phloretin and maltose as competitive inhibitors. J Physiol 278:389–401
Bihler I, Cybulsky R (1973) Sugar transport at the basal and lateral aspect of the small intestinal cell. Biochim Biophys Acta 298:429–437
Ciaraldi TP, Olefsky JM (1983) Length of acute exposure to insulin regulates the rate of deactivation of stimulated glucose transport in isolated rat adipocytes. Endocrinology 113:1739–1745
Crofford OB, Renold AE (1965) Glucose uptake by incubated rat epididymal adipose tissue. J Biol Chem 240:14–21
Czaky TZ, Fischer E (1981) Intestinal sugar transport in experimental diabetes. Diabetes 30:568–574
Diamond JM, Karasov WH (1984) Effect of dietary carbohydrate on monosaccharide uptake by mouse small intestine in vitro. J Physiol 349:419–440
Fritzsch G, Haase W, Rumrich G, Fasold H, Ullrich KJ (1984) A stopped flow capillary perfusion method to evaluate contraluminal transport parameters of methylsuccinate from interstitium into renal proximal tubular cells. Pflügers Arch 400:250–256
Frohnert PP, Höhmann B, Zwiebel R, Baumann K (1970) Free flow micropuncture studies of glucose transport in the rat nephron. Pflügers Arch 315:66–85
Gallagher BM, Fowler JS, Gutterson NI, McGregor RR, Wan CN, Wolf AP (1978) Metabolic trapping as a principle of radiopharmaceutical design: Some factors responsible for biodistribution of [18F] 2-deoxy-2-fluoro-d-glucose. J Nucl Med 19:1154–1161
Gatley SJ, Holden JE, Halama JR, De Grado TR, Bernstein DR, Ng CK (1984) Phosphorylation of glucose analog 3-O-methyl-d-glucose by rat heart. Biochem Biophys Res Comm 119:1108–1014
Glossmann H, Neville DM Jr (1972) Phlorrhizin receptors in isolated kidney brush border membranes. J Biol Chem 247:7779–7789
Hopfer U, Sigrist-Nelson K, Amman E, Murer H (1976) Differences in neutral amino acid and glucose transport between brush border and baso-lateral plasma membrane of intestinal epithelial cells. J Cell Physiol 89:805–810
Joost HG, Göke R (1984) Effects of islet activating protein on insulin- and isoprenaline-stimulated glucose transport in isolated rat adipocytes. FEBS Letters 167:5–9
Kahlenberg A, Dolansky D (1972) Structural requirements ofd-glucose for its binding to isolated human erythrocyte membranes. Can J Biochem 50:638–643
Kellett GL, Jamal A, Robertson JP, Wollen N (1984) The acute regulation of glucose absorption, transport and metabolism in rat small intestine by insulin in vivo. Biochem J 219:1027–1035
Kinne R, Murer H, Kinne-Saffran E, Thees M, Sachs G (1975) Sugar transport by renal plasma membranes vesicles. Characterization of the system in the brush border microvilli and basal-lateral plasma membranes. J Membrane Biol 21:375–395
Kleinzeller A, Kolinská J, Beneš I (1967) Transport of monosaccharides in kidney-cortex cells. Biochem J 104:852–860
Kleinzeller A, McAvoy EM (1977) Transport and metabolism ofd-glucose in renal tubular cells. Proceedings of the Int. Union of Physiol. Sciences, vol XIII, XXVII. Int Congr of Physiol Sciences, 18–23 June, Paris, p 391
Löschke K, Baumann K, Renschler H, Ullrich KJ, Fuchs G (1969) Differenzierung zwischen aktiver und passiver Komponente desd-Glucosetransports am proximalen Konvolut der Rattenniere. Pflügers Arch 305:118–138
Milla E (1980) Tubular extrusion ofd-glucose in the isolated rabbit kidney perfused in vitro. Pflügers Arch 388:29–35
Milla E (1983) Time-dependent and other possible changes of the intracellulard-glucose and Na+ concentration in the cortical tissue of rabbit kidney perfused “in vitro”. Pflügers Arch 398:55–59
Misfeldt DS, Sanders MJ (1981) Transepithelial transport in cell culture:d-glucose transport by pig kidney cell line (LLC-PK1). J Membrane Biol 59:13–18
Moran A, Turner RJ, Handler JS (1984) Hexose regulation of sodium hexose transport in LLC-PK1 epithelia: The nature of signal. J Membrane Biol 82:59–65
Pessin JE, Lillotson LG, Isselbacher KJ, Czech MP (1984) Photoaffinity labelling of the stereospecificd-glucose transport system with cytochalasin B. Fed Proc 43:2258–2261
Silverman M (1974) The chemical and steric determinants governing sugar interactions with renal tubular membranes. Biochim Biophys Acta 332:248–262
Silverman M (1980) Participation of the ring oxygen in sugar interaction with transporters at renal tubular surfaces. Biochim Biophys Acta 600:502–512
Silverman M, Turner RJ (1982) 2-Deoxy-d-glucose transport in dog kidney. Am J Physiol 242:F711-F720.
Suzuki K, Kono T (1980) Evidence that insulin causes translocation of glucose transport activity to plasma membrane from an intracellular storage site. Proc Natl Acad Sci USA 77:2542–2545
Tillotson LG, Yamada K, Isselbacher KJ (1984) Regulation of hexose transporters of chicken embryo fibroblast during glucose starvation. Fed Proc 43:2262–2264
Turner RJ, Silverman M (1978) Sugar uptake into brush border vesicles from dog kidney. I. Specificity. Biochim Biophys Acta 507:305–321
Turner RJ, Moran A (1982) Heterogeneity of sodium-dependentd-glucose along the proximal tubule. Evidence from vesicle studies. Am J Physiol 242:F406-F414
Ullrich KJ, Frömter E, Hinton BT, Rumrich G, Kleinzeller A (1976) Specificity of sugar transport across the brush border of the rat proximal tubule. In: Schmidt U, Dubach UC (eds) Current problems in clinical biochemistry: 6. Renal metabolism in relation to renal function. Hans Huber, Bern Stuttgart Wien, pp 256–261
Ullrich KJ, Rumrich G, Klöss S (1974) Specificity and sodium dependence of active sugar transport in the proximal convolution of the rat kidney. Pflügers Arch 351:35–48
Ullrich KJ, Fasold H, Rumrich G, Klöss S (1984) Secretion and contraluminal uptake of dicarboxylic acids in the proximal convolution of rat kidney. Pflügers Arch 400:241–249
Ullrich KJ, Rumrich G, Klöss S (1984) Contraluminal sulfate transport in the proximal tubule of the rat kidney. I. Kinetics, effects of K+, Na+, Ca2+, H+ and anions. Pflügers Arch 402:264–271
Ullrich KJ, Rumrich G, Klöss S (1985) Contraluminal transport of paraaminohippurate in the proximal convolution of rat kidney (in preparation)
Vansteveninck J, Weed RI, Rothstein A (1965) Localization of erythrocyte membrane SH groups essential for glucose transport. J Gen Physiol 48:617–632
von Baeyer H, von Conta C, Haeberle D, Deetjen P (1973) Determination of transport constants for glucose in proximal tubules of the rat kidney. Pflügers Arch 343:273–286
Wright EM, Van Os CH, Mircheff AK (1980) Sugar uptake by intestinal basolateral membrane vesicles. Biochim Biophys Acta 597:112–124
Zipper H, Mawe RC (1974) The exchange and maximal net flux of glucose across the human erythrocyte. II. The effect of two sulfhydryl enzyme inhibitors, chlormerodrin and p-chloromercuribenzene sulfonic acid. Biochim Biophys Acta 356:207–218
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ullrich, K.J., Papavassiliou, F. Contraluminal transport of hexoses in the proximal convolution of the rat kidney in situ. Pflugers Arch. 404, 150–156 (1985). https://doi.org/10.1007/BF00585411
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00585411